Rabies Vaccine Serial Numbers
Definition
Aug 15, 2017 - Guidelines and Rules for the Administration of Animal Rabies Vaccine.
Rabies vaccine is an injection that provides protection against the rabies virus that can be transmitted to humans via the saliva of an infected animal. Rabies is fatal in humans unless it is prevented with a vaccine.
Description
Rabies are caused by viruses of the genus Lyssavirus in the family Rhabdoviridae. Although all mammals are thought to be susceptible to rabies infection, the primary hosts are carnivores and bats. Most human exposure to rabies occurs via an animal bite in which the skin is broken and the virus is transmitted from the infected animal's saliva to the blood and tissues of the victim. The rabies virus infects the human nervous system causing acute encephalomyelitis, an inflammation of the brain and spinal cord. Death, usually by respiratory failure, occurs within seven to 10 days after appearance of the first symptoms. The average incubation period before symptoms of the disease appear is three to seven weeks, with a range of 10 days to seven years.
Prevalence of rabies
UNITED STATES Cases of human rabies are very rare in the United States due to the routine vaccination of domestic animals. In the past most human rabies resulted from bites by infected dogs. However as the incidence of rabies in dogs has decreased dramatically, rabies among wildlife has increased across the continental United States. Bat bites are now the most common source of human rabies infection. Hawaii remains rabies-free.
Between 1990 and 2003, there were 39 diagnosed cases of rabies among Americans. Every year an estimated 18,000 Americans receive rabies pre-exposure prophylaxis and an additional 16,000-39,000 receive post-exposure prophylaxis as a result of animal bites.
WORLDWIDE Rabies is common in some parts of the world, particularly in the developing countries of Africa, Asia, and Latin America. Rabies has been eradicated in the United Kingdom. Rabies is considered to be a reemerging viral disease because it is poorly controlled in many developing countries despite widely available human and animal vaccines. WHO estimates that every year about 10–12 million people worldwide receive post-exposure prophylaxis and that about 35,000 people—primarily children—die of rabies every year. However the incidence of rabies in the developing world is believed to be severely underreported. Most rabies exposures are from bites by unvaccinated dogs.
Vaccine development
The French scientist Louis Pasteur developed the first vaccine against rabies. In 1885, he injected his attenuated (weakened) virus into a nine-year-old boy who had been bitten by a rabid dog. The child's life was saved. Over the following century several generations of rabies vaccines were developed.
Although there is no cure for rabies once symptoms of the disease have appeared, in the 1980s scientists developed a highly effective vaccine that provides protection from the virus both before exposure—pre-exposure prophylaxis—or after exposure—post-exposure prophylaxis. The vaccine consists of killed rabies virus that, when injected, induces the child's immune system to produce antibodies that bind to and destroy the virus. The antibody response develops within seven to 10 days of vaccination and provides protection for up to two years. A second type of rabies vaccine, rabies immune globulin (RIG), provides immediate, short-term protection after exposure to the virus.
Pre-exposure prophylaxis
Routine rabies vaccination and booster immunizations are necessary only for those in high-risk professions such as veterinarian medicine and laboratory workers. However pre-exposure prophylaxis of children who are at risk of being exposed to rabid animals eliminates the need for RIG and decreases the number of required vaccinations after exposure. Pre-exposure prophylaxis is particularly important for children who may be exposed to rabies in places where vaccines, if available, may cause adverse reactions. Pre-exposure prophylaxis also may be helpful for children who are exposed unknowingly or do not report the exposure.
Children traveling internationally are at particular risk for rabies exposure because they may not exhibit caution in approaching animals. Such children may be considered for pre-exposure prophylaxis if they will be:
- in an area where rabies is prevalent or endemic
- camping in rural areas
- in an area where appropriate rabies vaccines and RIG may not be available
Post-exposure prophylaxis
After exposure to a potentially rabid animal, a child's risk of contracting rabies is assessed based on:
- the rabies vaccination status of the animal
- the type of animal
- whether the animal can be captured and tested for rabies
- the geographical location of the exposure
- whether the contact was provoked or unprovoked Unprovoked attacks are more likely to come from a rabid animal. Provoked attacks can include bites received while feeding or handling an animal.
Post-exposure prophylaxis usually is recommended when a child has been:
- bitten by any animal, including a pet dog or cat, that has not been vaccinated against rabies
- scratched or bitten by a wild animal, particularly a bat, raccoon, skunk, fox, or coyote (Some animals, particularly bats, may not leave obvious bite marks.)
When a child is bitten by a healthy domestic dog, cat, or ferret, the animal is usually confined for 10 days and observed for signs of rabies prior to initiating post-exposure prophylaxis. The rabies status of an animal also can be determined by testing for antibodies against rabies in its blood or by killing the animal and testing its brain tissue.
Post-exposure prophylaxis should be considered following any contact between a child and a bat, even if there is no evidence of a bite or scratch, since the child may be unaware of the contact and marks may not be apparent. For example, post-exposure prophylaxis should be considered if an unattended child is found in a room with a bat and the bat cannot be tested for rabies.
Vaccine types
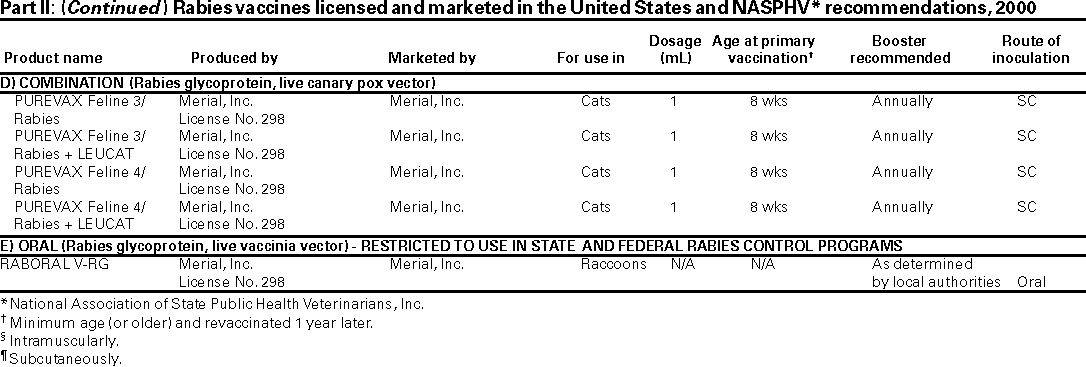
Four formulations of three inactivated rabies virus vaccines are licensed for use by the U.S. Food and Drug Administration (FDA). Two RIG formulations are also FDA-licensed.
HUMAN DIPLOID CELL VACCINE (HDCV) Human diploid cell vaccines (HDCVs) use inactivated rabies viruses. HDCV comes in two formulations: one for intramuscular (IM) injection and one for intradermal (ID) injection into a deep layer of skin.
PURIFIED CHICK EMBRYO CELL VACCINE (PCEC) Purified chick embryo cell (PCEC) vaccine became available in the United States in 1997. PCEC is made from rabies virus grown in cultures of chicken embryos and then inactivated. The drug is formulated for IM administration only.
RABIES VACCINE ADSORBED (RVA) Rabies vaccine adsorbed (RVA) is manufactured from virus grown in cell cultures of fetal rhesus monkey lung cells and then inactivated.
RABIES IMMUNE GLOBULIN (RIG) Human rabies immune globulin (RIG, HRIG) is a vaccine made from human serum that contains high levels of antibodies against rabies. It is used in conjunction with an inactivated-rabies vaccine for post-exposure prophylaxis. RIG provides immediate but short-lived protection against rabies. Approximately one-half of the antibodies are lost within 21 days after administration.
RIG is separated from the blood plasma of hyperimmunized human donors. Numerous procedures are used to clear the serum of rabies virus.
OTHER VACCINES Although the four types of inactivated-rabies vaccines and the two RIGs are the only rabies vaccines available in the United States, various other rabies vaccines are produced throughout the world. Although inactivated-rabies vaccines from diploid cell cultures are safe and effective, they are expensive. In developing countries, rabies vaccines often contain nerve tissue which can cause adverse effects. Various less expensive but safe and effective vaccines are under development.
General use
Inactivated-rabies vaccines are injected, either before or after exposure to the virus, in 1.0-ml. doses containing at least 2.5 IU/ml. of rabies virus antigen. This is the recommended standard of the World Health Organization (WHO). The size and number of vaccine doses are the same for children and adults. Although the same rabies vaccine usually is used throughout an immunization series, there is no evidence of adverse reactions or loss of effectiveness when two different vaccines are used in the same series. Modern rabies vaccines are relatively painless.
Pre-exposure prophylaxis
For preventative rabies immunization in an unexposed child, an inactivated-rabies vaccine is administered in three 1.0-ml. doses, with the second dose seven days after the first, and the third dose 21 or 28 days after the first. The vaccine is injected into the upper arm. Studies have found that this regimen produces adequate antibodies against rabies in the blood serum of all subjects.
Post-exposure prophylaxis
Following an animal bite or contact between a child's mucous membranes and an animal's saliva, an attempt is usually made to determine whether the animal has rabies. If there is a threat of rabies, an unvaccinated child receives RIG and a series of five rabies vaccinations over a 28-day period. Ideally, treatment should begin within two days of exposure, however it may be started at any time thereafter.
The wound is cleaned thoroughly and, if possible, RIG is injected into the wound and the surrounding tissues to block the virus's entry into the central nervous system. The recommended dose is 20 IU/kg (1 kg = 2.2 lb) of body weight. This is equivalent to 22 mg of the antibody immunoglobulin G (IgG) per kilogram of body weight. Any remaining RIG is injected intramuscularly at a site removed from the vaccination site. RIG also may be injected into the buttocks. RIG is never injected with the same syringe or at the same site as the vaccine. RIG is used only once to provide antibodies until the child's immune system begins producing its own antibodies in response to the vaccine. RIG is administered concurrently with the first dose of inactivated-rabies vaccine or up to seven days thereafter. Additional treatment with RIG may interfere with antibody production in response to the inactivated-rabies vaccine.
Inactivated-rabies vaccine is administered in 1.0-ml. doses, at three, seven, 14, and 28 days after the first vaccination. It is injected intramuscularly in the upper arm or the upper thigh. If an animal is found to be rabies-free after the vaccination series has been initiated, the series can be discontinued.
Exposure following vaccination
Children exposed to rabies following vaccination receive a 1.0-ml. dose of vaccine immediately and a second dose three days later. These children do not receive RIG because it will diminish the rapid antibody response resulting from the previous vaccination.
Precautions
Precautions should be taken before vaccinating a child who has:
- a weakened immune system due to HIV/AIDS or other disease or condition
- had a life-threatening reaction to a previous rabies vaccine or to any component of the vaccine
Children with suppressed immune systems should not receive pre-exposure prophylaxis against rabies. Medical conditions and medications that suppress the immune system can interfere with antibody production in response to a rabies vaccine. If a child has exhibited a serious hypersensitivity to a previous rabies vaccine, antihistamines may be used concurrently. Children who are allergic to eggs should not be give vaccines cultured in chicken embryos.
A minor illness, such as a cold, does not preclude rabies vaccination. However pre-exposure vaccination should be postponed if the child has a moderate or severe illness. Post-exposure prophylaxis should be administered regardless of any other illness or condition. Children should not be vaccinated against measles or chickenpox (varicella) for four months after being treated with RIG. Children receiving post-exposure prophylaxis outside of the United States should have their antibody levels against rabies measured after their return.
Side effects
Side effects from the rabies vaccines currently used in the United States are much less common and less severe than the side effects of earlier rabies vaccines. New edition discography 320kbps torrent. However side effects may vary with the brand of vaccine and adverse reactions to rabies vaccines used in some other countries are quite common. The risk of side effects also increases with the number of vaccine doses. However a vaccination series should not be interrupted because of localized or mild side effects.
Mild side effects from rabies vaccines include:
- soreness, redness, swelling, itching , or pain at the site of the injection in 30–74 percent of recipients
- headache, nausea , abdominal pain, muscle aches, or dizziness in 5–40 percent of recipients
More serious side effects of rabies vaccines include:
- hives, joint pain, or fever in about 6 percent of those receiving a booster vaccination
- very rarely, an illness resembling Guillain-Barré syndrome, a disorder of the motor nerves that can result in temporary paralysis, lasting no longer than 12 weeks and resulting in complete recovery Other nervous system disorders occur so rarely following rabies vaccination that they may not be related to the vaccine. However, a physician should be consulted if a high fever or behavioral changes occur following rabies vaccination.
Reported side effects of RIG include:
- local pain
- low-grade fever
Although any vaccine is capable of inducing an allergic reaction, serious reactions to rabies vaccine are very rare. Signs of an allergic reaction include:
- paleness
- weakness
- dizziness
- hoarseness or wheezing
- difficulty breathing
- a fast heartbeat
In case of a serious reaction to a rabies vaccine:
- A doctor should be consulted immediately.
- The date, time, and type of reaction should be recorded.
- Medical personnel or the local health department should file a Vaccine Adverse Event Report.
Interactions
Immune system-suppressing treatments, including cancer drugs and radiation and steroids, can interfere with the antibody response to rabies vaccination. If possible, immunosuppressive medications should be suspended during the vaccination series, and the vaccine injections should be intramuscularly. Alternatively, the child's serum can be checked for antibody production to determine if the vaccination was successful.
Chloroquine phosphate or similar anti-malarial drugs such as mefloquine may interfere with the response to HDCV. Children who will be taking anti-malarial drugs while traveling in areas with endemic rabies should begin the three-dose regimen of ID vaccine one month prior to travel, before they begin taking drugs to prevent malaria. However, a three-dose, pre-exposure regimen of IM vaccine provides an adequate response even in the presence of anti-malarial drugs.
Preparing a child for an injection
Most children are afraid of injections; however there are simple methods for easing a child's fear . Prior to the vaccination parents should:
- Tell children that they will be getting a shot.
- Explain to children that the shot will prevent them from becoming sick.
- Have older siblings comfort and reassure a younger child.
- Bring along the child's favorite toy or blanket.
- Never threaten children by telling them they will get a shot.
- Read the vaccination information statement (VIS) and ask questions of the medical practitioner.
During the vaccination parents should:
- Hold the child.
- Make eye contact with the child and smile.
- Talk softly and comfort the child.
- Distract the child by using a hand puppet or pointing out pictures or objects.
- Sing or tell the child a story.
- Have the child tell a story.
- Teach the child to focus on something other than the shot.
- Help the child take deep breaths.
- Allow the child to cry.
- Stay calm.
After the injection
Following an injection parents should:
- Hold and caress a child or breastfeed an infant.
- Talk soothingly and reassuringly.
- Hug and praise the child for doing well.
- Use a cool, wet cloth to reduce soreness or swelling at the injection site.
Parents should also be aware that:
KEY TERMS
Antibody —A special protein made by the body's immune system as a defense against foreign material (bacteria, viruses, etc.) that enters the body. It is uniquely designed to attack and neutralize the specific antigen that triggered the immune response.
Antigen —A substance (usually a protein) identified as foreign by the body's immune system, triggering the release of antibodies as part of the body's immune response.
Booster immunization —An additional dose of a vaccine to maintain immunity to the disease.
Encephalomyelitis —Encephalitis or another acute inflammation of the brain and spinal cord that can be caused by the rabies virus.
Human diploid cell vaccine (HDCV) —A rabies vaccine in which the virus is grown in cultures of human cells, concentrated, and inactivated for IM or ID injection.
Intracutaneous —Into the skin, in this case directly under the top layer of skin.
Intramuscular(IM) —An injection into a muscle.
Prophylaxis —Protection against or prevention of a disease. Antibiotic prophylaxis is the use of antibiotics to prevent a possible infection.
Purified chicken embryo cell vaccine (PCEC) —A rabies vaccine in which the virus is grown in cultures of chicken embryo cells, inactivated, and purified for IM injection.
Rabies immune globulin (RIG or HRIG) —A human serum preparation containing high levels of antibodies against the rabies virus; used for post-exposure prophylaxis.
Rabies virus adsorbed (RVA) —A rabies vaccine in which the virus is grown in cultures of lung cells from rhesus monkeys, inactivated, and adsorbed to aluminum phosphate.
- The child may eat less during the first 24 hours following a vaccination.
- The child should drink plenty of fluids.
- The medical practitioner may suggest a non-aspirincontaining pain reliever for the child.
BOOKS
Jackson, Alan C., and William H. Wunner, eds. Rabies. Boston: Academic Press, 2002.
Sep 25, 2012 - We are also looking: product key bitdefender 2012 crack keygen, reason 6 pc full keygen crack username password serial, eset nod32 version. Feb 5, 2018 - PASSWORD Forza Motorsport 4 2011 [PC Windows Full Game] Cracked DOWNLOAD. Forza motorsport 4 crack and keygen serial 2011 for pc - Forza 4 mod tools Nov 16, 2011-4 minForza MotorSport 3 Mod Tool V3. With keygen crack serial.  Jan 15, 2018 - Download Forza Motorsport 4 from Follow the Steps Given Below to Download Redeem Codes for Forza Motorsport 4 STEP 1 - Download.
Jan 15, 2018 - Download Forza Motorsport 4 from Follow the Steps Given Below to Download Redeem Codes for Forza Motorsport 4 STEP 1 - Download.
PERIODICALS
Kammer, A. R., and H. C. Ertl. 'Rabies Vaccines: From the Past to the 21st Century.' Hybrid Hybridomics 21, no. 2 (April 2002): 123-7.
ORGANIZATIONS
Centers for Disease Control and Prevention. 1600 Clifton Rd. NE, Atlanta, GA 30333. (800) 311-3435. Web site: http://www.cdc.gov.
Immunization Action Coalition. 1573 Selby Ave., St. Paul, MN 55104. (651) 647-9009. Web site: http://www.immunize.org.
WEB SITES
'About RabAvert.' RabAvert Rabies Vaccine. Chiron Corporation. 2001 [cited August 10, 2004]. Available online at: http://www.rabavert.com/about.html.
CDC's Rabies Web Page That's Just for Kids! Centers for Disease Control and Prevention. February 6, 2002 [cited July 27, 2004]. Available online at: http://www.cdc.gov/ncidod/dvrd/kidsrabies/Vaccination/Vaccination.htm.
'First Rabies Cases from Organ Transplant Reported.' Reuters Health. July 1, 2004 [cited July 27, 2004]. Available online at: http://www.nlm.nih.gov/medlineplus/news/fullstory_18699.html.
'Human Rabies Prevention—United States, 1999 Recommendations of the Advisory Committee on Immunization Practices (ACIP).' Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention. January 8, 1999 [cited August 9, 2004]. Available online at: http://www.cdc.gov/epo/mmwr/preview/mmwrhtml/00056176.htm.
Rabies. Centers for Disease Control and Prevention. July 9, 2004 [cited July 27, 2004]. Available online at: http://www.cdc.gov/ncidod/dvrd/rabies.
Rabies Vaccine Serial Number
'Rabies.' Travelers' Health. Centers for Disease Control and Prevention. April 12, 2004 [cited July 27, 2004]. Available online at: http://www.cdc.gov/travel/diseases/rabies.htm.
Rabies Number Lookup
Rabies Prevention and Control. National Center for Infectious Diseases. December 1, 2003 [cited July 27, 2004]. Available online at: http://www.cdc.gov/ncidod/dvrd/rabies.
Nobivac Rabies Manufacturer
Rabies Vaccine: What You Need to Know. National Immunization Program. November 4, 2003 [cited August 5, 2004]. Available online at: http://www.cdc.gov/nip/publications/VIS/vis-rabies.pdf.
Sherman, Max. 'Rabies: A History and Update on Prophylaxis Regimens in the U.S.' U.S. Pharmacist. [cited August 10, 2004]. Available online at: http://www.uspharmacist.com/oldformat.asp?url=newlook/files/Feat/aug00rabies.cfm&pub_id=8&article_id=563.